Creon®
Mode of Action
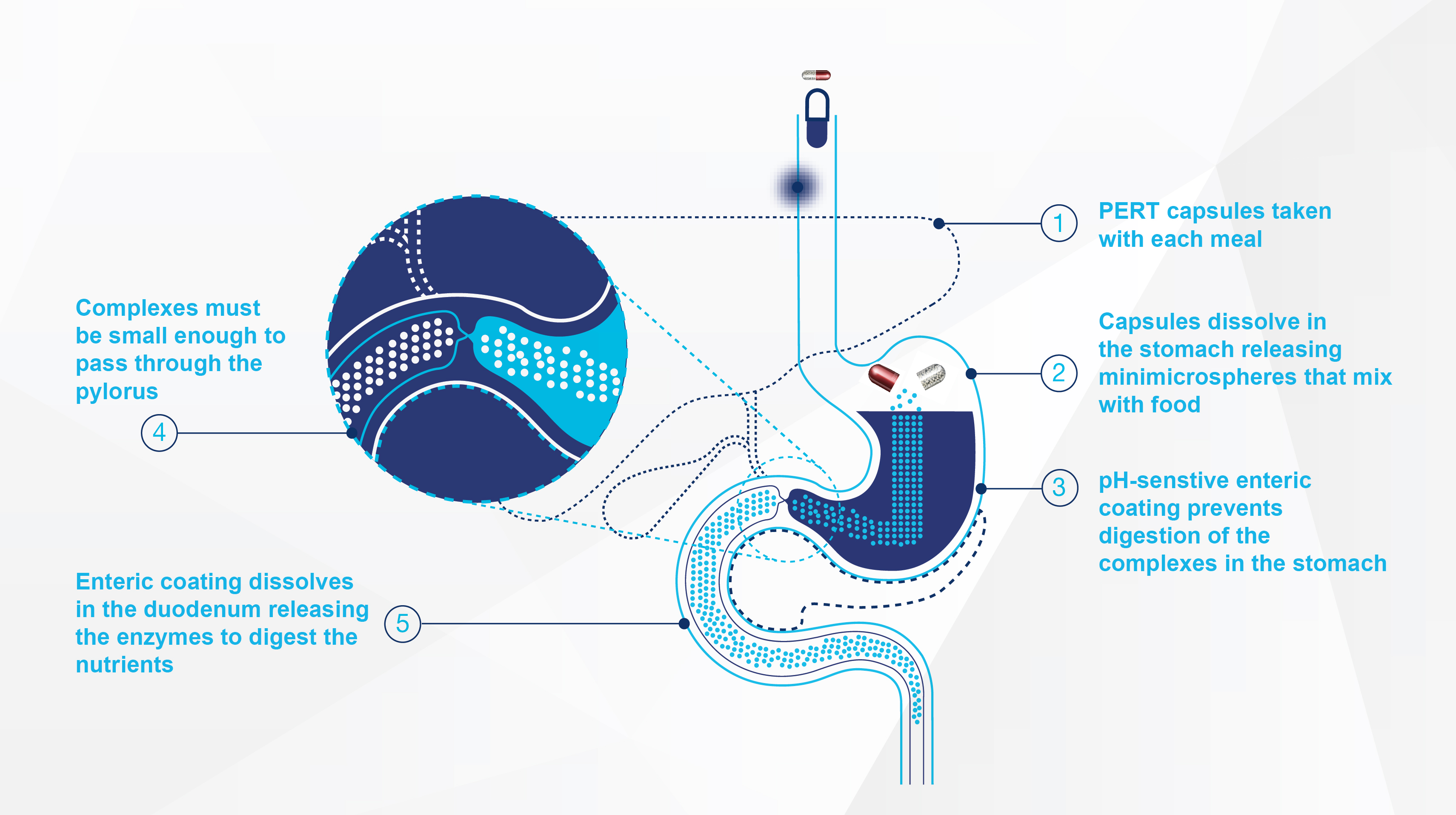
Unique MINIMICROSPHERES® technology
Creon® has a unique enteric-coated MINIMICROSPHERES® technology with particle sizes of 0.7–1.6 mm in diameter.1-3
The pH-sensitive enteric coating prevents gastric acid from denaturing the lipase.4 Once the intact enzymes reach the alkaline pH of the duodenum, the enteric coating rapidly dissolves to release the enzymes into the lumen.5
How the MINIMICROSPHERES® technology works
Watch the video to see how Creon® works
References
- Colombo C et al. Efficacy and Tolerability of Creon for Children in Infants and Toddle with Pancreatic Exocrine Insufficiency caused by Cystic Fibrosis. Pancreas, 2009, 38(6):693-699.
- Löhr JM et al. Properties of different pancreatin preparations used in pancreatic exocrine insufficiency. Eur J Gastroenterol. Hepatol. 2009, 21:1024-1031.
- Domínguez-Muñoz JE. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency: when it is indicated, what is the goal and how to do it?. Adv Med Sci. 201; 56(1):1-5.
- Struyvenberg MR, et al. Practical guide to exocrine pancreatic insufficiency - Breaking the myths. BMC Med. 2017;15(1):29.
- 2015 Australasian Pancreatic Club. Australasian guidelines for the management of pancreatic exocrine insufficiency. https://static1.squarespace.com/static/5b5e94e7aa49a1a136248110/t/5db0c976c894034e9ce620b8/1571867020139/APC+Guidelines+on+PEI.pdf
CREON® (Pancreatic Extract). Modified release capsules: General Sale (10,000 IU lipase) & Prescription Medicine (25,000 IU lipase); Modified release granules: General Sale (Micro 5,000 IU lipase). Indications: For treatment of conditions associated with pancreatic exocrine insufficiency (PEI), such as: cystic fibrosis (CF), chronic pancreatitis, post-pancreatectomy, post gastrointestinal bypass surgery, ductal obstruction of the pancreas or common bile duct. Contraindications: Hypersensitivity to porcine protein or to any of the excipients. Precautions: Fibrosing colonopathy, pregnancy, lactation. Adverse Effects: Abdominal pain, nausea, vomiting, constipation, diarrhoea, abdominal distension, rash, pruritus, urticaria. Dosage & Administration: The dose required depends on the severity of disease and the composition of food. It is recommended to take the enzymes during or immediately after each meal or snack. Always ensure adequate hydration of patients to avoid constipation. The capsules should be swallowed intact however when swallowing of capsules is difficult, the capsules may be opened and the minimicrospheres added to acidic soft food such as apple sauce, yoghurt or fruit juice with pH < 5.5, or taken with liquid such as fruit juice with pH < 5.5, e.g. apple, orange or pineapple juice. Rinse mouth out afterwards to ensure no product is retained in the mouth. Treatment of adult patients with PEI associated with nonCF conditions – Required dose for meals: 25,000 to 80,000 lipase units; Snacks: half of meal dose. Refer to data sheet for dose titration considerations. Treatment of paediatric and adult patients with CF - Children < 4 years: Starting dose of 1,000 lipase units/kg bodyweight per meal; Patients ≥ 4 years: Starting dose of 500 lipase units/kg bodyweight per meal. For CF patients, maximum dose of 4,000 lipase units/gram dietary fat intake. Creon® is a fully funded medicine. Before recommending this medicine, please refer to the full Data Sheet available from www.medsafe.govt.nz. Creon® and MINIMICROSPHERES® are Viatris company trade marks, Viatris Limited, Auckland. CRE-2023-0643. TAPS DA 2303MM-0712.